Nitric acid 70%, purified by redistillation, ≥99.999% trace metals basis - 1 X 475 mL
Nitric acid 70%, purified by redistillation, ≥99.999% trace metals basis - 1 X 475 mL
Synonym(s): Aqua fortis, Azotic acid, Hydrogen nitrate
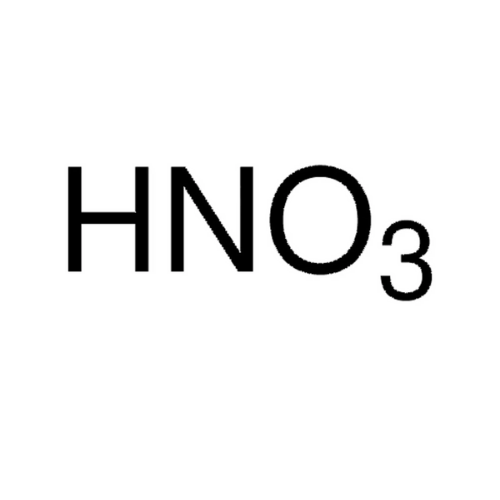
Empirical Formula (Hill Notation): HNO3
CAS Number: 7697-37-2
Molecular Weight: 63.01
Nitric acid is generally used as a primary reagent for the nitration of organic molecules. It is also used as a strong oxidizing agent in organic synthesis to perform the oxidative transformations.[1][2][3]
Application
Nitric acid used as a reagent in:
• The nitration of aromatic compounds, aromatic heterocycles, alkenes, active methylene carbons, etc.[1][4]
• Selective oxidation of organosulfur compounds to corresponding sulfoxides using a solid acid catalyst.[5]
• The oxidation of alcohols, aldehydes, esters, and heteroatoms.[1]
• The aromatization and dehydrogenation reactions.[1]
It's Our Responsibility

Small choices. Big impact.
Science moves us forward. So should sustainability. At Go Green Scientific, we offer carefully selected lab supplies designed to reduce waste and protect the planet—without compromising quality or compliance.
Science Meets Sustainability
We know scientists ask tough questions—and we welcome them. That’s why we vet every product for performance, sustainability, and integrity.