Nitric acid puriss. p.a., 65.0-67.0% - 1 X 1 L
Nitric acid puriss. p.a., 65.0-67.0% - 1 X 1 L
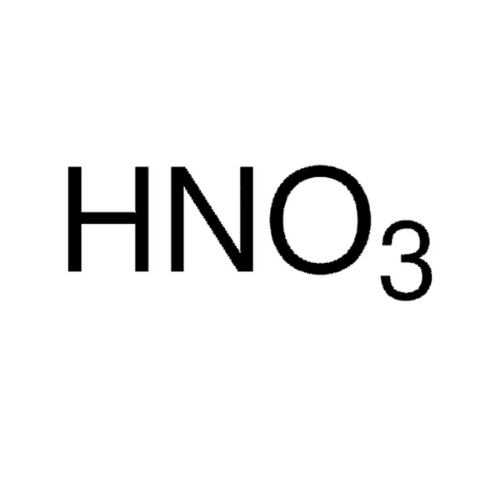
Empirical Formula (Hill Notation): HNO3
CAS Number: 7697-37-2
Molecular Weight: 63.01
General description
Nitric acid (HNO3) is a highly corrosive strong mineral acid. In HNO3, nitrogen element exhibits +5 oxidation state.[1] 10%(v/v) nitric acid solution is useful for cleaning laboratory glasswares.[2] Reaction of HNO3 with various original and synthetic mineral dust/mineral oxide surfaces was studied in a low-pressure Knudsen reactor operating at 298K.[3]
Application
Nitric acid (HNO3) may be used in the following studies:
• Oxidation of multiwalled carbon nanotubes (MWCNTs).[4]
• Removal of transition metal catalyst from the single-walled carbon nanotubes (SWNTs) during the purification of SWNTs.[5]
• Trace determinations of Pb and Cd in aqueous medium by flame atomic absorption spectrometry.[2]
• Preparation of lead dioxide (PbO2).[6]
Used for the decomposition of organic and inorganic samples for det. of mercury
It's Our Responsibility

Small choices. Big impact.
Science moves us forward. So should sustainability. At Go Green Scientific, we offer carefully selected lab supplies designed to reduce waste and protect the planet—without compromising quality or compliance.
Science Meets Sustainability
We know scientists ask tough questions—and we welcome them. That’s why we vet every product for performance, sustainability, and integrity.